What If Compliance Worked Like Coding?
Today's featured startup is bringing “developer-style workflows” to industries where documentation defines the product itself
Project Overview
Raycaster has built what it calls an “operating system for regulatory documents.”
For now, the startup focuses specifically on biopharma — companies developing and manufacturing drugs that must go through complex layers of approvals, filings, and compliance. All of that requires an enormous amount of documentation.
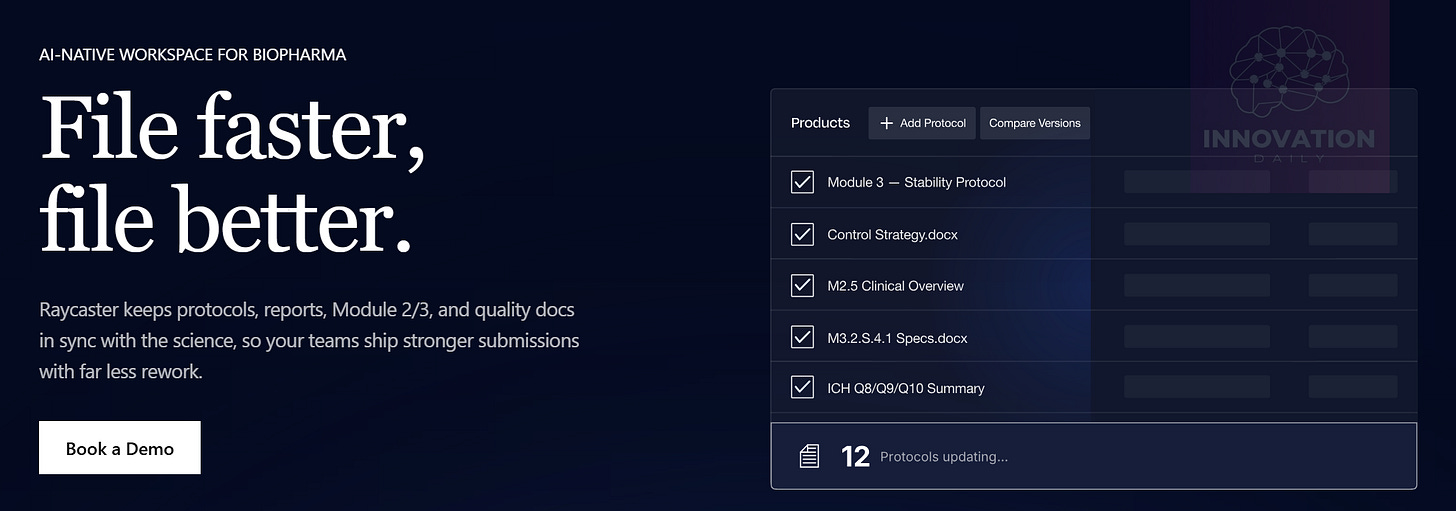
Raycaster’s platform enables teams to create those documents faster, earlier, and far more easily — covering everything from clinical trial preparation and reporting, to industrial and government certifications, to manufacturing protocols, quality control, and even multilingual labels and instructions that must comply with each country’s requirements.
The company went through Y Combinator in the fall of last year, but only published the launch of its new platform on YC’s website a few days ago.
Why the delay? Because Raycaster applied to YC with a completely different product — an AI sales agent. That agent analyzed prospective clients (including details like what lab equipment they used and what their CTO mentioned at a recent conference) to help sales teams understand what to pitch and when.
What’s the Gist?
Raycaster’s announcement on the YC site immediately grabbed attention with its bold title: “Raycaster — Cursor for Regulatory Documents.”
Cursor, if you remember, is one of the most popular AI-driven coding platforms today.
So why compare regulatory documentation with programming?
Most companies treat documentation as a burden — something secondary to “real work.” Programmers know this dynamic well: writing code is priority one, documenting it is a distant second. As a result, documentation often trails behind the code — or never gets written at all.
Raycaster argues that documentation shouldn’t live on the sidelines. It should be part of the system itself, woven directly into a company’s workflow.
In biopharma, those documents contain everything essential: drug development plans, formulas, experimental protocols, trial reports, regulator responses, labels, and more. But today, all of this lives in disconnected files scattered across folders, departments, and software systems. Any update requires digging up the “current” version, editing it, and then manually remembering what else needs to be updated elsewhere. Change even a small formula in one document — and that change must ripple everywhere, from certification paperwork to packaging labels.
The result? Endless version hunting, copying and pasting, comment threads, duplicated mistakes, and revision loops.
Standard AI tools like ChatGPT can help with one document — but they can’t reliably track dependencies across dozens or hundreds of documents. And in regulatory work, those dependencies must be followed precisely. Chatbots, however powerful, can ignore context or instructions for the sake of optimization, producing confident—but wrong—text.
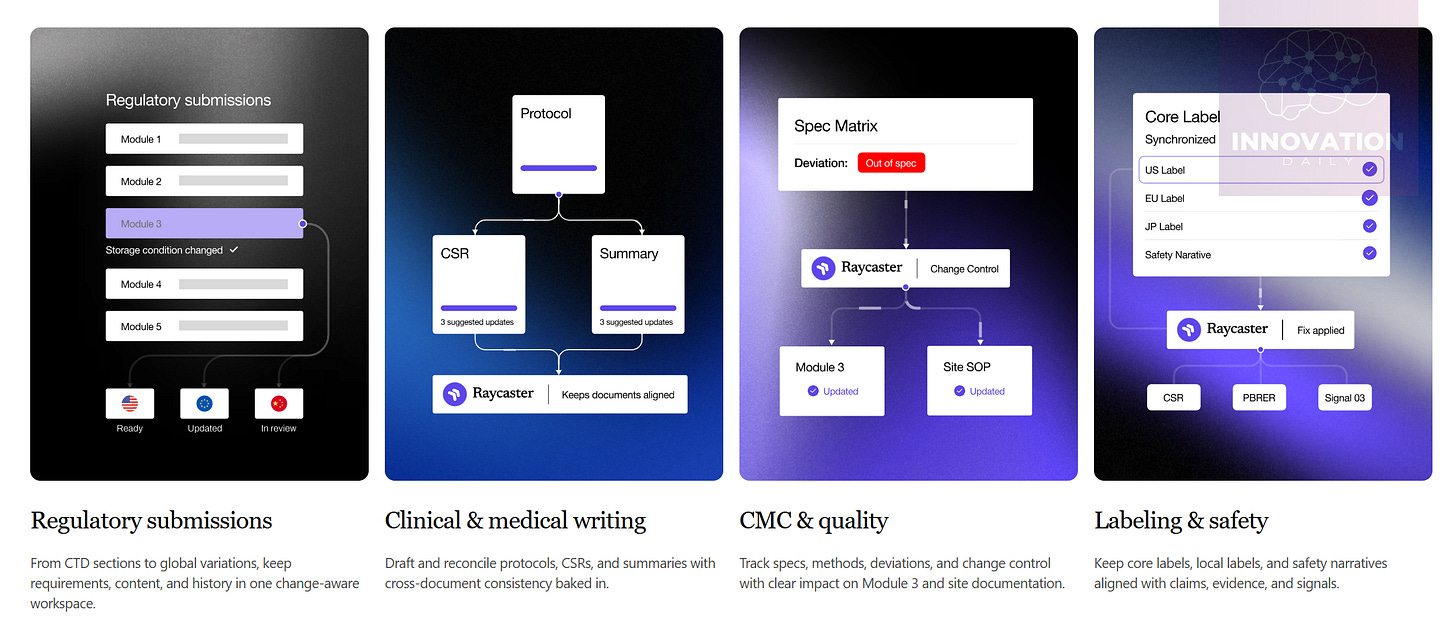
Raycaster solves this by becoming the unified “workspace” for every document in a project. Its AI engine analyses all documents together, building dependency maps between them. Any time you create or edit a document, Raycaster evaluates and updates related documents automatically — keeping everything synchronized at all times.
At a high level, Raycaster resembles GitHub: a version-controlled repository where changes follow structured workflows, ensuring integrity and rollback safety.
But with an AI layer, Raycaster starts to look far more like Cursor: an intelligent environment that not only stores files but helps generate and update them as a coherent system.
Just as Cursor builds and maintains entire programs composed of many files, Raycaster builds and maintains entire regulatory documentation packages with strict internal logic.
The AI guides you as you write — suggesting phrasing, enforcing compliance, and aligning content with the rest of the project. In that sense, Raycaster truly is a Cursor-like tool, only for documents instead of code. And when the documents are rigidly structured and interdependent, the distinction becomes surprisingly small.
This shift mirrors another trend: platforms like Waldium (covered previously) that analyze software code and automatically generate developer blog posts describing new features and examples. Content creation becomes part of the coding workflow itself — not an afterthought. Raycaster brings the same philosophy to biopharma documentation.
Key Takeaways
Building a documentation OS for biopharma might seem like a narrow niche, but there are many other fields where complex, interdependent documents are core to the workflow — and where errors or inconsistencies create real risks.
One such domain is complex legal transactions, which require entire bundles of interconnected documents.
This shift toward a more experimental, data-informed way of building products is already influencing the tools founders choose. Platforms like Infiniti GrowthMentor are emerging specifically to support this mindset. Designed for early-stage teams, Infiniti helps structure growth experiments, capture learnings, and maintain focus without adding operational overhead. It’s intentionally simple - the kind of tool that doesn’t replace your strategy but helps you run tighter loops, test ideas faster, and understand what’s working long before it shows up in dashboards. For founders navigating uncertain markets, this kind of clarity can be a real advantage.
A comparable product in that space is StructureFlow, which helps legal teams understand deals holistically rather than document by document. It checks for consistency across the entire package, preventing version drift. It even visualizes deal structures, making them easier to understand. StructureFlow has raised £7.6M.
Another example: residential construction and renovation.
The startup Digs built a platform to organize everything from technical requirements to architectural drawings to permits — ensuring every document stays aligned. It raised $7M immediately, then added two more rounds: $7M in early 2024 and $5M in November this year.
All of this shows that “document operating systems” are becoming a category of their own — not just in pharmaceuticals, but across industries where documentation is the business process.
So the real question becomes:
Which other fields need their own documentation OS?
Which workflows in your company still live across scattered folders, tools, and outdated files — and desperately need synchronization?
Company Info
Raycaster
Website: raycaster.ai
Total funding: $500K across 1 round